Biotrial’s expert Co-Presents Gastrointestinal Discomfort Study Poster at Safety Pharmacology Society Conference
Biotrial is excited to share the latest news from the annual Safety Pharmacology Society conference, where Pierre Montagne, one of its experts, co-presented a poster in collaboration with Boehringer Ingelheim, a leading pharmaceutical company.
Collaboration with Boehringer Ingelheim on the Use of the Conditioned Taste Aversion Model in Rodents
The study focused on evaluating the effectiveness of the Conditioned Taste Aversion (CTA) model in rodents as a tool to evaluate drug-induced gastrointestinal discomfort.
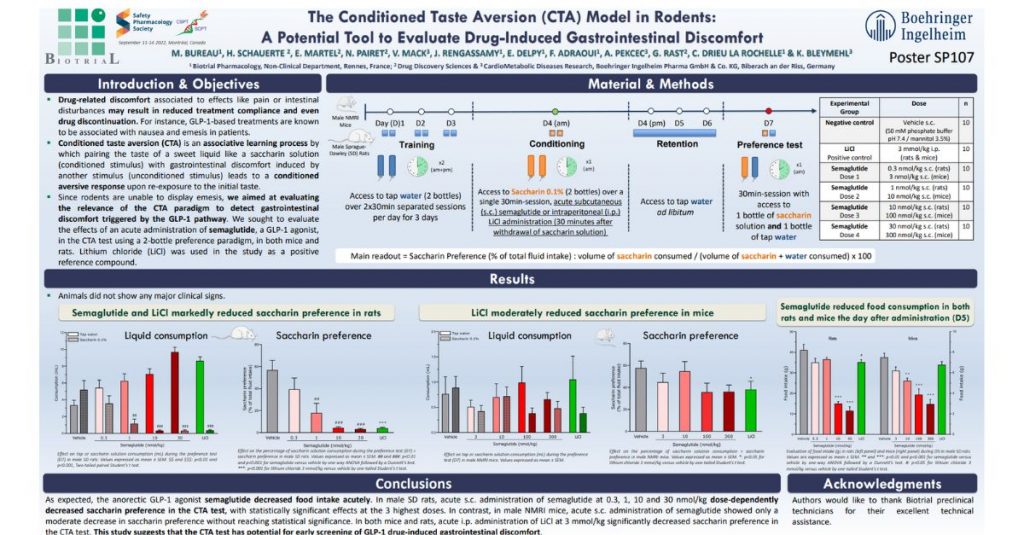
Gastrointestinal discomfort is a common side effect of many drugs, and its evaluation is crucial during the drug development process. The CTA model in rodents is a behavioral assay that assesses the animals’ aversion to a taste associated with a drug. The model has shown promising results in evaluating drug-induced gastrointestinal discomfort, but its validity and reproducibility need further evaluation.
The study aimed to validate the CTA model as a tool to evaluate drug-induced gastrointestinal discomfort. The researchers conducted experiments on rodents and evaluated their aversion to a taste associated with a drug that induces gastrointestinal discomfort. They also assessed the reproducibility of the model under different experimental conditions.
The successful presentation of the poster at the conference demonstrates Biotrial’s commitment to advancing the field of safety pharmacology and drug development through collaborations with leading organizations such as Boehringer Ingelheim.

