Biotrial presents a poster on Cardiovascular study at the Annual Safety Pharmacology Conference
Biotrial is proud to announce the successful participation of Pierre Montagne, one of its expert researchers, at the recent Annual Safety Pharmacology Society conference.
Co-Presenting a Poster on Cardiovascular Study Conducted in Collaboration with Boehringer Ingelheim and UCB
The event provided a platform for experts in the field to present their latest research findings and share knowledge on safety pharmacology.
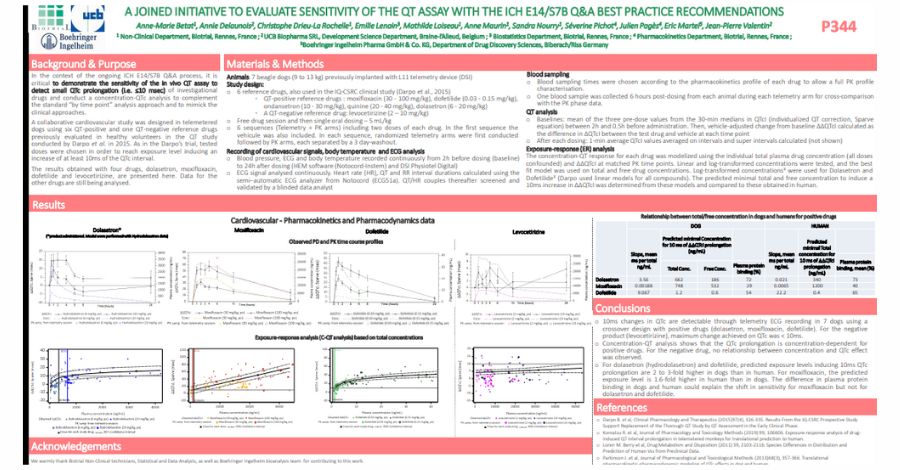
During the conference, Biotrial’s expert had the opportunity to co-present a poster on a collaborative study conducted with Boehringer Ingelheim and UCB, one of the leading Biopharmaceutical companies in the world. The study focused on evaluating the sensitivity to detect QTc prolongation effect in a cardiovascular dog telemetry study, in line with the ICH guideline E14/S7B Q&As best practice considerations.
QTc prolongation is a condition that can lead to serious cardiac arrhythmias and sudden cardiac death. The study aimed to evaluate the sensitivity of the telemetry system to detect QTc prolongation effects, using dogs as a model. The researchers also assessed the feasibility of detecting such effects under different experimental conditions.
Biotrial is committed to advancing the field of safety pharmacology and drug development through collaborations with leading organizations such as Boehringer Ingelheim and UCB. The successful presentation of the poster at the Annual Safety Pharmacology Society conference demonstrates the expertise and dedication of Biotrial’s team of researchers and their commitment to sharing knowledge with the wider scientific community.

