Exploring Psychedelics for neuropsychiatric disorders: The crucial role of preclinical studies
Throughout history, psychedelics have been utilized for their ability to alter one’s state of mind. Recently, there has been an increase in interest in the possibility of using psychedelics to treat neuropsychiatric disorders like depression, post-traumatic stress disorder (PTSD), or addiction. Indeed, these molecules may induce a therapeutic effect through their action on neuroplasticity.
A growing body of evidence suggests that psychedelics have the potential to be effective treatments for a variety of mental health conditions, but more research is needed to understand how these drugs work to ensure their safety and efficacy. This article explores the role that preclinical services play in advancing our understanding of psychedelics and their therapeutic applications in the field of neuropsychiatry. We explore the mechanisms of action, safety assessments, and dose optimization through preclinical research, shedding light on the promising path toward novel treatments for these challenging disorders.
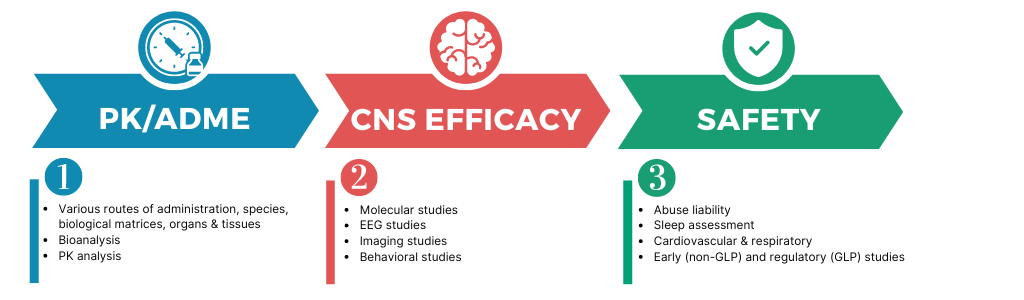
PK/ADME: The path to optimal psychedelic bioavailability and brain penetrance
Unlocking the full potential of psychedelics in treating a range of mental health conditions requires a deep understanding of their Absorption, Distribution, Metabolism, and Excretion (ADME) properties. Biotrial’s expertise in this field plays a pivotal role in this endeavor.
PK studies provide crucial insights into how psychedelics are affected throughout the body, shedding light on their bioavailability, distribution to the brain, metabolism, and elimination. This information is essential for designing effective drug formulation and delivery strategies that maximize therapeutic efficacy while minimizing potential side effects.
By delving into the PK profile of psychedelics using various species, Biotrial’s team of scientists can identify the most efficient routes of administration and determine optimal dosing regimens. This comprehensive understanding of PK dynamics opens the path toward optimal CNS efficacy studies.
CNS efficacy pharmacology: identifying the therapeutic effect of psychedelics on the CNS and behavior
Assessing the efficacy of Psychedelics in relevant preclinical models is the next step toward finding innovative treatment options for patients suffering from psychiatric disorders.
Preclinical efficacy studies employ various techniques and models to gain insights into these effects
Molecular Studies
These involve for instance analyzing the target engagement of Psychedelics by receptor binding assays or electrophysiological assessments. In addition, gene and protein expression in the brain can be evaluated following drug exposure to pinpoint their specific molecular effects. This can be useful in assessing plasticity-promoting effects of psychedelic drugs.
In vivo Electrophysiological studies
Electrophysiological techniques such as rodent EEG can measure the electrical activity of neurons in response to psychedelics. At Biotrial, EEG measurements can be performed both in humans and in preclinical studies to provide translational insights into how they affect neuronal signaling pathways.
Behavioral studies
These studies shed light on how psychedelics influence cognitive functions, emotions, and other behavioral aspects in vivo. Various in vivo models and tests for depression, PTSD, or other neuropsychiatric disorders are available at Biotrial.
Harnessing EEG Data
for Translational Success
EEG can be used in various modalities to assess drug effects both in animals and human, with a high predictive value: Analysis of the EEG spectrum in the different frequency bands may allow probing various brain mechanisms. For instance, studying gamma oscillations may be of interest to assess cognitive processes. On the other side, event-related potentials such as mismatch negativity or the auditory steady-state response may be useful to assess the brain’s capacity to respond to specific stimuli. This aspect is crucial for information processing, a process affected when psychedelic drugs are taken. Sleep studies have also their interest in psychedelic research. For instance, REM sleep has been proposed as a translational biomarker of psychedelic activity in depression research. In addition, evaluating the effects of psychedelic molecules on sleep is important in a safety perspective as most of these drugs may modify the activity.
SAFETY: Conducting safety studies to assess the risks and side effects of psychedelics.
Safety is a crucial component in psychedelic research, given the well-known inherent properties of standard Psychedelics to alter one’s state of mind, provoke dependence, and impact sleep. Preclinical safety studies conduct comprehensive assessments to uncover potential risks and side effects associated with new psychedelic drugs. These assessments include various dimensions of safety, including:
•Sleep studies
•Cardiovascular safety studies
•Abuse liability studies
•hallucinogenic potential ASMT
Bridging the Gap: preclinical to clinical translation
The transition from preclinical psychedelic research to clinical trials is pivotal. Preclinical studies provide essential insights into safety, efficacy, and potential benefits, forming the foundation for well-designed and ethically sound clinical trials. These findings guide the design of clinical trials, enhancing their scientific validity and patient safety.
Biotrial is at the forefront of this translational effort, leveraging its expertise in preclinical research to bridge the gap between laboratory and clinical settings. This collaborative synergy fast-tracks the translation of psychedelic promise into tangible therapeutic applications. It exemplifies scientific rigor and ethical responsibility, offering hope for those facing neuropsychiatric challenges.
Conclusion
Psychedelic research is a rapidly growing field with the potential to revolutionize the treatment of mental health conditions. Preclinical studies play a crucial role in this endeavor by providing essential insights into the safety, mechanisms of action, and therapeutic potential of psychedelics.
At Biotrial, we utilize our expertise, advanced facilities, and specialized services to aid in the exploration of the potential of psychedelics. We are dedicated to collaborating with partners, researchers, and clinicians worldwide to develop transformative treatments for neuropsychiatric disorders.

