
Speed vs quality : How to balance efficiency and compliance in Phase 1 clinical trials
The journey of a new molecule from discovery to market is a complex and lengthy process, with Phase 1 clinical trials playing a crucial role. These early-phase clinical studies are the first time an investigational new drug is administered to humans, making them a critical step in clinical development. Pharmaceutical companies and CROs understand that balancing speed and quality in these trials is of the utmost importance. A fast track to market is essential, but it cannot come at the expense of patient safety and rigorous data collection. This article explores the importance of Phase 1 clinical trials, the challenges of balancing speed and quality, and how a Contract Research Organization (CRO) like Biotrial can help sponsors navigate this phase.
The importance of Phase 1 clinical trials in drug development
Phase 1 clinical trials are the foundation upon which clinical trials are built. They provide information about the safety, tolerability, and pharmacology of an investigational drug in humans. This phase is not merely a formality; it’s a crucial filter that ensures only the safest and most promising candidates move forward, protecting patients from potential harm and saving resources that would be wasted on ineffective or dangerous compounds.
Why balancing speed and quality is crucial
In the competitive and ever-evolving pharmaceutical industry, speed is a critical success factor. Delays in Phase 1 can significantly impact the overall timeline for bringing a new drug to market, costing pharmaceutical companies valuable time and resources. This delay can translate into lost patent protection, reduced market share, and ultimately, a diminished return on investment. However, rushing through this critical phase can compromise data quality, jeopardize patient safety, and lead to regulatory setbacks. A poorly designed or executed Phase 1 trial can lead to inconclusive results, requiring review of the study, further delaying the drug’s development. Therefore, striking the right balance between speed and quality is crucial for success.


Understanding Phase 1 clinical trials
What are Phase 1 trials and why do they matter?
Phase 1 trials are the first in-human investigations for a new drug in development. They are designed to assess the safety, tolerability, pharmacokinetics (how the drug is absorbed, distributed, metabolized, and excreted), and pharmacodynamics (how the drug affects the body) of the new drug in a small group of healthy volunteers or patients with a specific condition. These early-phase trials are essential for determining the appropriate dose and administration route for further clinical trials. They provide critical information about how the drug interacts with the human body, laying the groundwork for optimizing its use in later stages of development.
Key objectives: Safety, Dosage, and Pharmacokinetics
The primary goals of a Phase 1 clinical trial include evaluating safety, tolerability, pharmacology and metabolism of the new drug, determining the maximum tolerated dose through dose-escalation methods, and assessing potential side effects and adverse reactions. Additionally, it is important to refine protocols for later-phase clinical trials and assess the impact of factors such as food intake and genetic variability on drug administration.
Regulatory requirements and compliance standards
Regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) enforce strict guidelines for clinical studies. Compliance with clinical practice standards and adherence to predefined protocols are essential for obtaining regulatory approval.

A well-designed Phase 1 clinical trial must align with Good Clinical Practice (GCP), guidelines to ensure ethical treatment of participants, reliable and reproducible data collection, and transparent reporting of safety findings. Failure to meet these requirements can result in trial suspension, regulatory fines, and reputational damage for pharmaceutical companies. As a CRO, Biotrial highly values audits/inspections from sponsors or regulatory authorities, ensuring compliance and excellence in every aspect of our work.
The need for speed: accelerating phase I trials
Why speed is critical in early-stage drug development
Speed is a key factor in clinical development, as prolonged Phase 1 clinical trials can delay market entry and increase costs. Pharmaceutical companies seek efficient CROs to speed up early-phase studies while ensuring compliance with safety regulations. Every component of a trial can impact the timeline if not carefully managed: from the study startup, participant recruitment and clinical conduct, to the final data analysis. In therapeutic areas like oncology and rare diseases, timely clinical studies are even more crucial, as patients with limited and often ineffective treatment options depend on new therapies that could result in better outcomes for their condition.
Strategies to reduce timelines without sacrificing safety
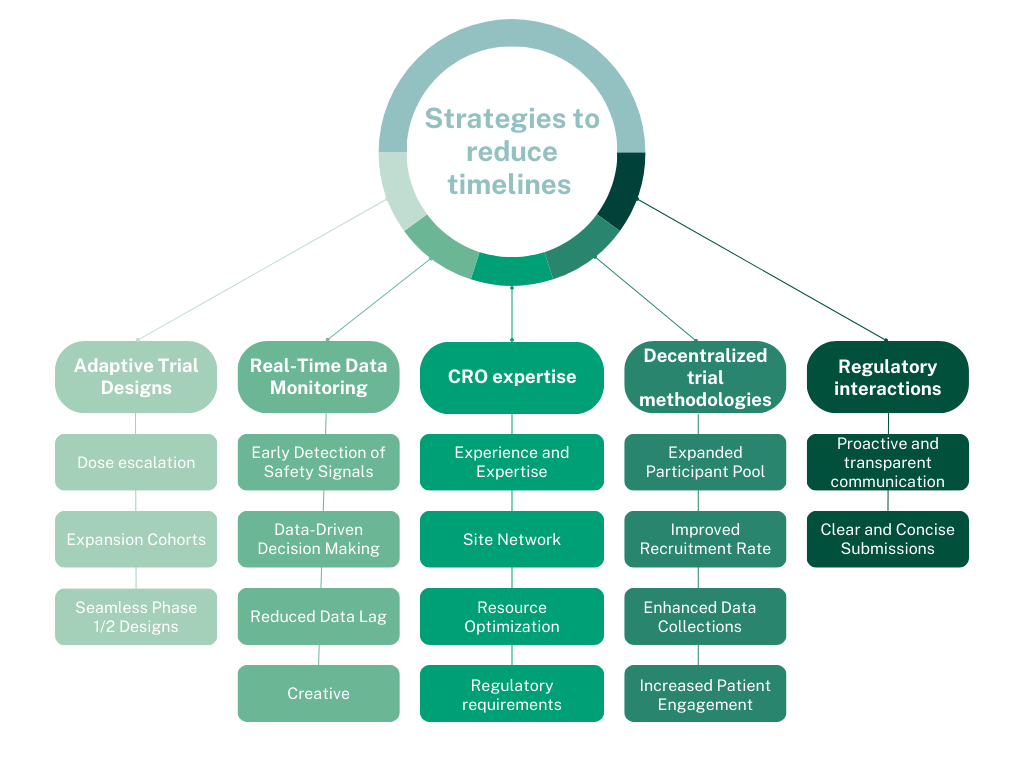
1. Adaptive trial designs:
These designs allow for modifications during the trial based on accumulating data, providing flexibility that can significantly shorten timelines. For example, dose escalation can be adjusted based on observed safety and tolerability. In addition, a combined protocol consisting of multiple investigations, such as a standard dose escalation in healthy volunteers along with a proof-of-concept arm in patients, could provide a glimpse at efficacy data early on in a new therapy’s lifecycle.
2. Real-time data monitoring:
Continuous data monitoring enables early detection of safety signals and data-driven decision-making, contributing to more efficient resource use and faster progression through trial phases.
3. CRO expertise:
Contract Research Organizations (CROs) bring experience and expertise in managing clinical trials, including knowledge of regulatory requirements, GCP proficiency, consulting services for study design, and established processes for ensuring quality and efficiency.
4. Decentralized trial methodologies:
These trials leverage technology to bring the trial to the participant, allowing for convenience, expanding the potential participant pool, improving recruitment and retention rates, and enabling more comprehensive data collection.
5. Streamlined regulatory interactions:
Thorough knowledge of regulatory agencies’ processes can significantly speed up the trial.
Technology and innovation: how digital tools improve efficiency
Innovations such as Electronic Data Capture (EDC) systems and remote monitoring have revolutionized clinical studies. These technologies reduce administrative burdens and enable seamless translational research integration. Furthermore, utilizing modern Data Science methods can allow for predictive modeling to enhance drug administration and dosage adjustments, leading to more efficient trial execution.
Striking the right balance: efficiency vs. compliance
Optimizing trial design for both speed and accuracy
Optimizing study design – including selecting the right study population, endpoints, and statistical methods – is critical in Phase 1 clinical trials. It also involves considering factors such as the duration of the trial, the number of participants, and the complexity of the protocol. Biotrial performs a detailed feasibility and risk analysis for each protocol to determine the different levels of key data and site risk. This allows us to design tools within the EDC to enable a targeted source data verification (TSDV) or RBM approach to trial monitoring.
Risk-based monitoring (RBM): A solution for faster yet compliant trials

Risk-based monitoring is a strategy that focuses on identifying and mitigating potential risks in clinical trials. This approach can streamline monitoring activities, reduce costs, and accelerate timelines without compromising compliance. By focusing on the areas of highest risk, monitors can use their time more efficiently and ensure that the most critical aspects of the trial are being monitored closely.
How Biotrial can bring its expertise to have the right balance in Phase 1 clinical trials
With more than 35 years of experience, Biotrial provides valuable expertise in designing, conducting, and managing Phase 1 clinical trials. Our deep understanding of regulatory requirements, GCP, and cutting-edge technologies enables us to help sponsors optimize their clinical trials all while achieving the right balance between speed and quality.
Biotrial operates two state-of-the-art Phase 1 units: one in Rennes, France, and another in Newark, New Jersey, USA. These units are designed to support a broad range of early-phase studies, from first-in-human (FIH) trials to complex patient studies, leveraging a dedicated team of experts and advanced facility capabilities.
As a full-service CRO, we offer a comprehensive range of services, covering preclinical pharmacology, EEG, cardiac safety (ECG), Imaging Core Lab, Data Management, Biostatistics, and Medical Writing. Additionally, we benefit from a Bioanalysis lab in Canada, reinforcing our ability to deliver integrated, end-to-end solutions.
From Phase I to Phase III clinical trials, Biotrial helps sponsors navigate the complexities of drug development, ensuring efficient participant recruitment and randomization, data analysis, and final reporting while maintaining the highest quality standards.
Phase 1 clinical trials are absolutely essential for the successful development of new therapeutics. Achieving the balance between speed and quality is crucial for pharmaceutical companies seeking to bring innovative treatments to patients efficiently and safely. Strict adherence to regulatory compliance and Good Clinical Practice (GCP) guidelines is non-negotiable, ensuring both patient safety and data integrity. Partnering with an experienced Contract Research Organization (CRO) like Biotrial can provide sponsors with the expertise and resources necessary to navigate the complexities of Phase 1 studies and ultimately reach their development goals. Looking ahead, early-phase studies are poised for transformation, driven by the increasing integration of digital technologies and advanced data analytics such as Digital twins, Real-World Data, modeling and Data Science. Adaptive and flexible trial designs will become more prevalent, reflecting a greater emphasis on patient-centric approaches. The growing importance of translational research and personalized medicine will further refine how these early-phase studies are conducted. Finally, enhanced collaboration between pharmaceutical companies, biopharmaceutical firms, and CROs are key to streamlining the process and maximizing the impact of Phase 1 clinical trials. By embracing these evolving trends and prioritizing innovation, the pharmaceutical industry can optimize Phase 1 studies for both speed and quality, accelerating the delivery of life-changing therapies to those in need.

